Phases I, II and IIIa/b, Study design, Sample size, Statistical Analysis Plans, Interim Analysis..
RWE/RWD generation, Late Phase study design, Observational studies, Claims Databases, Patient Registries...
Data-Generation for Data Mining, Post-hoc analyses, Scientific & Medical material generation..
CRF/eCRF design, Study databases build, CDISC standards, Edit checks, QC and validation...
SAS 9.3/9.4, R, SDTM and ADaM standards, Mock tables, TFLs productions, QC ...
The critical step in any research project is identifying the initial clinical question. Once the objectives are precisely defined, a robust statistical methodology as well as a specific selection criteria for the study population can be defined, guaranteeing optimal final results. Signfience provides services in :
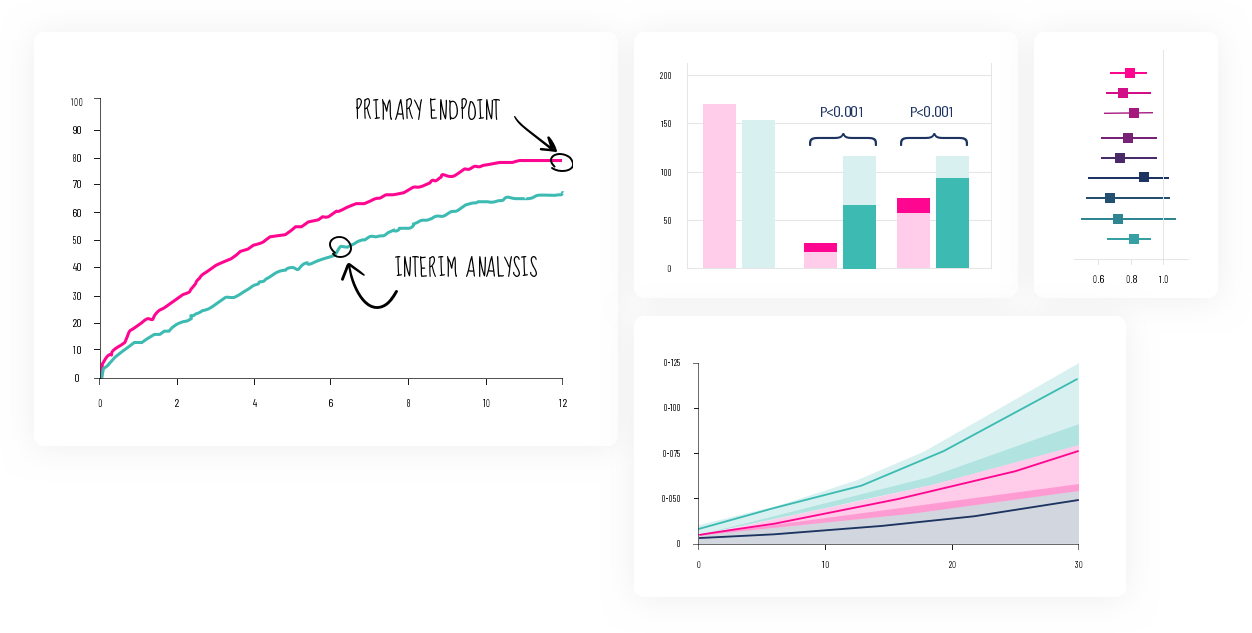

The evolution of technology being utilized in the exploration of clinical data is accelerating. Novel methods of Machine and Deep Learning offer exciting new opportunities in data analyses and scientific discoveries. This scientific revolution created by Artificial Intelligence is part of the DNA of Signfience.
The communication of study results to the scientific community and health authorities is a crucial step in the valorization of a research project.
Thanks to its team of experts in biometrics and programming, Signifience is committed to the production of precise and clear communication documents allowing information sharing and quality scientific publication:
